Written by: Sydney Wyatt
Edited by: Jennifer Baily and Will Louie
If you’re like me, you’ve probably been panic-refreshing the Yolo County website for
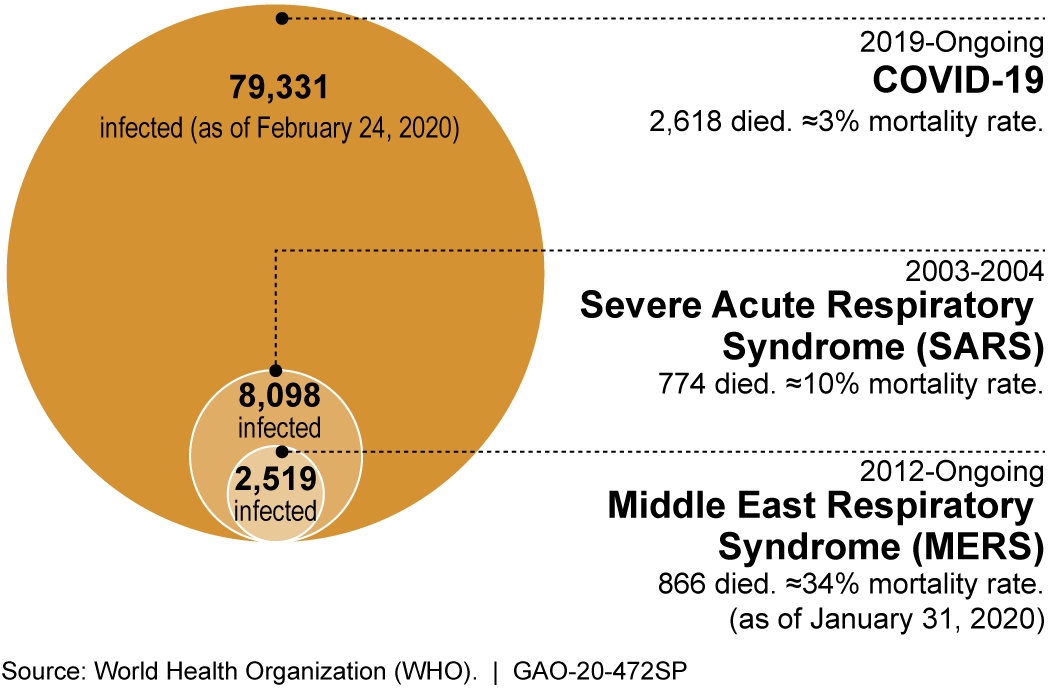
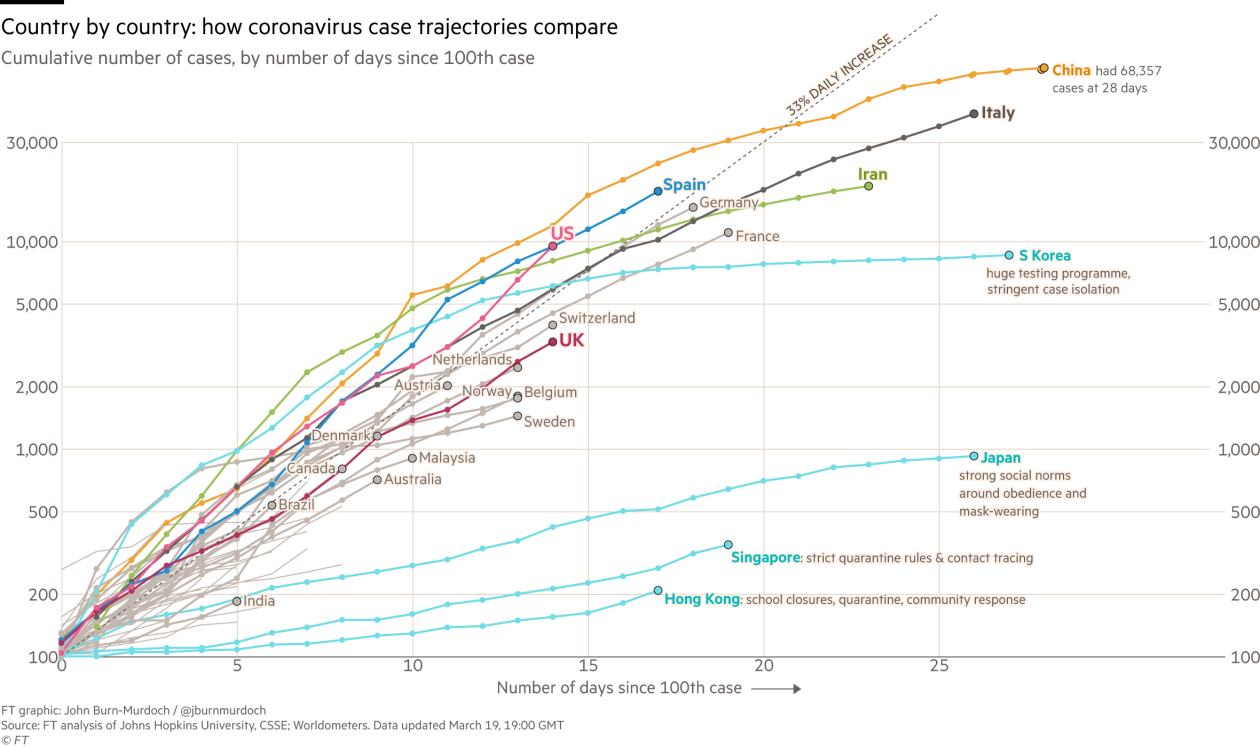
the newest case numbers as the COVID-19 pandemic continues. Case numbers continue to rise as testing capacity slowly increases, but social distancing appears to be flattening the curve in California. Disturbingly, there is still a huge backlog of samples to be tested and, currently, a high false negative rate.
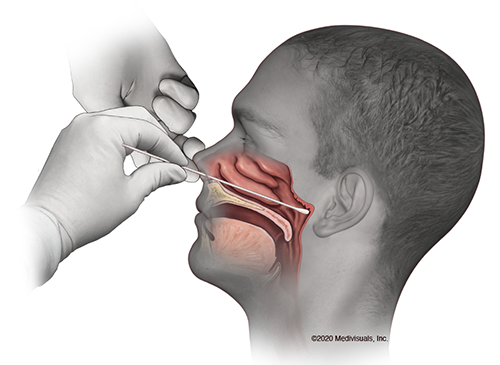
A nasal or throat swab is performed to get testable samples; according to one patient it “feels like they are swabbing your brain.” This sample is then shipped off to a laboratory to run a test to detect SARS-CoV-2 RNA. This involves RNA extraction, cDNA library prep, and finally RT-PCR to produce a band on an agarose gel that indicates a positive result. There is another method, approved by the FDA on March 13, that performs all these steps within a single automated instrument, but there is still doubt about whether it is better at extracting RNA without damage.
The FDA recently approved the use of Cellex’s antibody test, however antibody tests are not the best tool to diagnose illness. Antibodies develop to recognize and fight an invader, in this case the novel coronavirus; thus antibodies can only be detected during the late stages of symptomatic infection or after symptoms subside. It could help determine how many people actually had COVID-19, regardless if they tested negative or didn’t meet the testing requirements, however there is still debate whether antibody testing really works and demonstrates immunity. Antibody testing in California started on April 10. As of April 18, reports suggest that significantly more people have tested positive for antibodies than expected based on the laboratory test numbers.
While the initial World Health Organization (WHO) test implemented in January worked well for many countries, the Centers for Disease Control and Prevention (CDC) created its own test based on detecting different SARS-CoV-2 genes. While this test was shipped out starting February 6 after the CDC announced the first case on January 21, person-to-person spread was already occurring according to a CDC announcement on January 30 . The test was released under the Food and Drug Administration’s (FDA) emergency use authorization, meaning it was not vetted by the FDA prior to release. However, problems with the negative control were reported as early as February 12. Labs found that the primers for the negative control were binding to each other to produce a false positive. The initial test batches with the faulty primers were recalled from general use, resulting in a bottleneck at the CDC lab in Atlanta, Georgia. The CDC approved modification of the test on February 26 on a lab-by-lab basis, although the test remains imperfect and there is a significant backlog due to the delay. This delay in testing between evidence of community spread on January 30th and the authorization of test adjustment on February 26th put the US almost a month behind on keeping the virus in check.
While some county websites report the number of negative laboratory tests — like Santa Clara County in the Bay Area — there is currently a high false negative rate. Some reports indicate a 30% chance of a false negative, meaning 30% of those “negative” patients may actually have COVID-19. In some hotspots, this could significantly increase the number of positive cases. This inaccuracy can be caused by errors acquiring samples or during the testing process. The sample may not have enough genetic material. RNA is unstable and degrades easily, and the test process is difficult to troubleshoot due to complex steps. The antibody test may also give false negatives because not every patient will develop an antibody response as in the case of immunocompromised patients. RT-PCRs are notoriously finicky, yet they’re still the most sensitive testing method compared to antibody testing or culturing samples.
As of April 2, there were a reported 60,000 pending tests in California alone; Quest Diagnostics — one of the largest private testing facilities in the country — reported a backlog of 80,000 tests on April 13. Again, these are RT-PCR tests that must be performed at private labs like Quest Diagnostics or university labs because the equipment is not widely available at the point of care. The testing bottleneck is further dependent on machine time and reagent availability. Accelerated results require the development of a sensitive sample-to-answer test that can be run at the point of care, similar to getting a flu test at the doctor’s office.
A new point-of-care test from Abbott claims to detect infection in just 5 minutes using a specific proprietary machine already used in doctor’s offices across the country. It is important to note this test is also authorized by the FDA under emergency use authorization and will cease to be used when the authorization is revoked. Because it has not been officially vetted by the FDA, there are still doubts about its sensitivity.
While the backlog has been somewhat alleviated with 150,000 tests run per day last week, let’s keep it that way by staying home and physically distancing ourselves. Check out our articles about coronaviruses and maintaining your mental health while sheltering in place.